What is homeopathy?
Homeopathy is an alternative medical practice that was developed in the late 1700s. Homeopathy is generally based on two main principles:
- that a substance that causes symptoms in a healthy person can be used in diluted form to treat symptoms and illnesses, a principle known as “like-cures-like”; and
- the more diluted the substance, the more potent it is, which is known as the “law of infinitesimals.”
Historically, homeopathic products have been identified through “provings,” in which substances are administered to healthy volunteers in concentrations that cause symptoms. Symptoms experienced by volunteers are recorded to indicate possible therapeutic uses for the substances. In other words, if a substance causes a particular symptom, individuals experiencing that symptom would be treated with a diluted solution made from that substance.
There are no FDA-approved products labeled as homeopathic; this means that any product labeled as homeopathic is being marketed in the U.S. without FDA evaluation for safety or effectiveness.
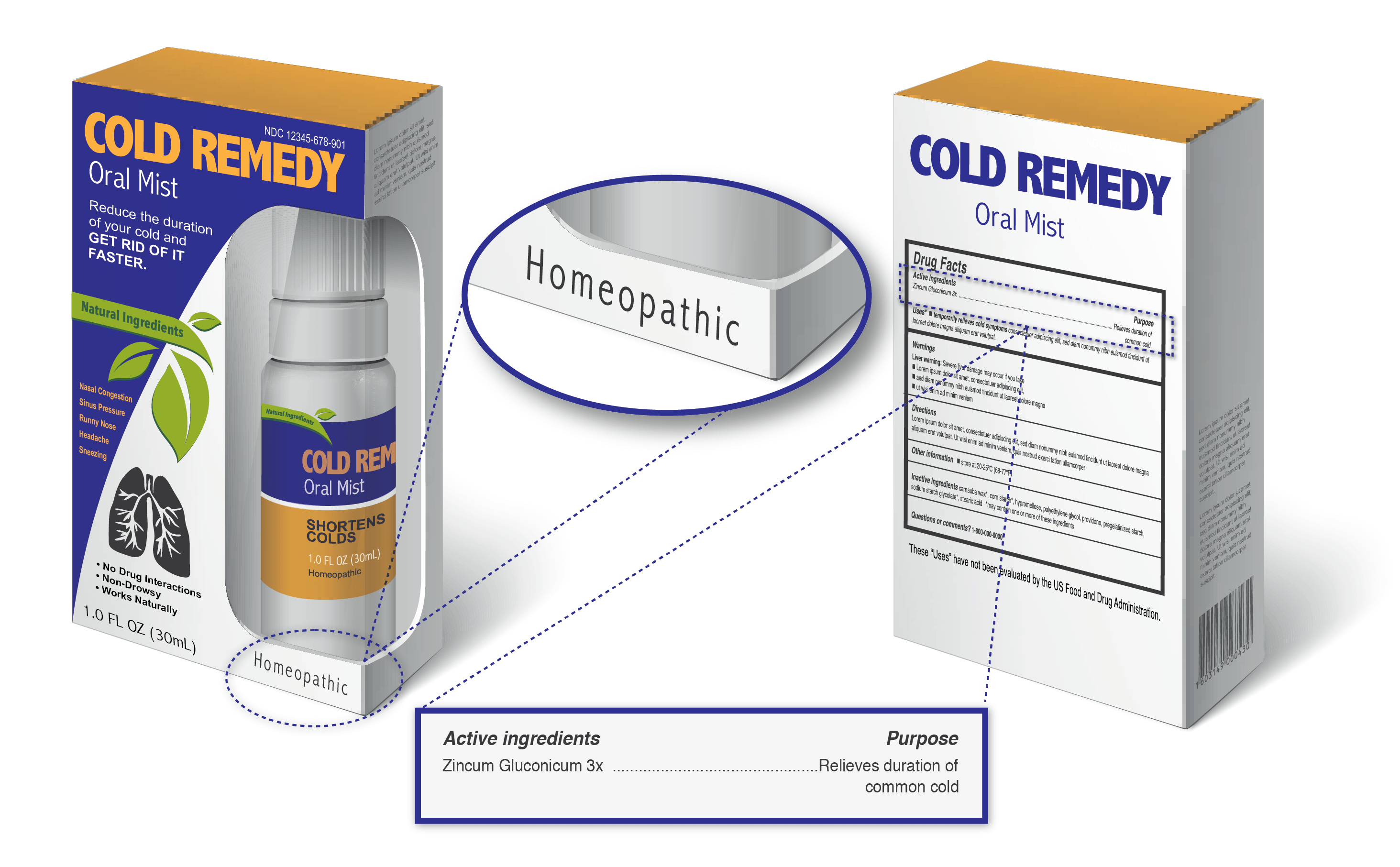
What are homeopathic products and how can you tell you are taking one?
These products are often marketed as natural, safe and effective alternatives to approved prescription and nonprescription products.
Products labeled as homeopathic can contain a wide range of substances, including ingredients derived from plants, healthy or diseased animal or human sources, minerals, and chemicals.
Products labeled as homeopathic generally include:
- The word “Homeopathic”
- The ingredients listed in terms of dilution, e.g. 1X, 6X, 2C
Is FDA concerned about the safety of homeopathic products?
While products labeled as homeopathic are generally labeled as highly diluted, some of these products have been found to contain measurable amounts of active ingredients and therefore could cause significant patient harm
FDA tested products that were improperly manufactured, which can cause incorrect dilutions and increase the potential for contamination. Further, some products labeled as homeopathic are marketed to treat serious diseases or conditions. Examples of FDA’s concerns, include:
- Letter from FDA to American Institute of Homeopathy regarding the “Certified Homeopathic” Seal
- FDA alerts consumers of Kadesh Incorporation’s voluntary nationwide recall of Puriton Eye Relief Drops due to non-sterile production conditions
- FDA alerts consumers of Sprayology’s voluntary nationwide recall of homeopathic water-based medicines due to microbial contamination
- FDA alerts consumers and pet owners of Silver Star Brand’s voluntary recall of homeopathic drug products
- FDA alerts consumers of HelloLife’s voluntary recall of Neuroveen, Respitrol, Thyroveev and Compulsin
- FDA alerts consumers of Beaumont Bio Med’s voluntary recall of all water- and alcohol-based products
- FDA alerts consumers of BioLyte Laboratories voluntary recall of NeoRelief
- FDA alerts consumers, pet owners not to use products manufactured by King Bio, including Dr. King’s label, homeopathic drug and pet products
- FDA warns consumers about homeopathic teething products
- FDA warns consumers about the potential health risks of over-the-counter asthma products labeled as homeopathic
- Information on Zicam Cold Remedy Nasal Gel, Zicam Cold Remedy Nasal Swabs, and Zicam Cold Remedy Swabs, Kids Size
- Pleo Homeopathic Drug Products by Terra-Medica: Recall - Potential for Undeclared Penicillin
What should consumers know about homeopathic products?
Products labeled as homeopathic and currently marketed in the U.S. have not been reviewed by the FDA for safety and effectiveness to diagnose, treat, cure, prevent or mitigate any diseases or conditions.FDA’s evidence-based drug reviews play an essential role in ensuring that drugs are made with quality manufacturing processes, and are safe and effective for their intended uses. Products that have not been evaluated for safety and effectiveness may harm consumers who choose to treat serious diseases or conditions with such products, and consumers may be foregoing treatment with a medical product that has been scientifically proven to be safe and effective.
FDA recommends consumers talk to their doctor or health care professional about safe and effective treatments for their disease or condition.
How are homeopathic products regulated?
Under the Federal Food, Drug, and Cosmetic Act, homeopathic products are subject to the same requirements related to approval, adulteration and misbranding as other drug products. There are currently no homeopathic products approved by FDA.
On December 6, 2022, FDA issued a final guidance, Homeopathic Drug Products, that describes the agency’s approach to prioritizing regulatory actions for homeopathic products posing the greatest risk to patients. Since homeopathic drug products have not been approved by FDA for any use, they may not meet modern standards for safety, effectiveness, and quality.
This guidance proposes a comprehensive, risk-based enforcement approach to homeopathic products marketed without FDA approval.
FDA’s approach will prioritize regulatory and enforcement actions involving unapproved homeopathic products that pose the greatest risk to patients. Many homeopathic products fall outside the risk-based categories described in the guidance. FDA intends to focus its enforcement authorities on the following kinds of products:
- with reported safety concerns;
- that contain or purport to contain ingredients associated with potentially significant safety concerns;
- for routes of administration other than oral or topical, e.g. for use as an injection or taken nasally;
- that claim to treat or prevent serious and/or life-threatening diseases and conditions, such as cancer;
- marketed to vulnerable populations, including children, pregnant women and the elderly; or
- with significant quality issues.
In 1988, the FDA issued Compliance Policy Guide (CPG) 400.400, “Conditions Under Which Homeopathic Drugs May be Marketed,” which described the agency’s enforcement policy. On October 24, 2019, FDA withdrew CPG 400.400 because it was inconsistent with the agency’s risk-based approach to regulatory and enforcement action.
Formore information
- FDA Drug Approval Process Infographic